Despite your best efforts, if you’re active in social media, you’ve been drawn into pointless conversations. Indeed, that’s why many people stay away. But if you can tolerate a little noise, I’ve found the fruitful far outweighs the fruitless. Case in point:
LinkedIn Member 1: “This looks like a great resource. I just hope they are planning on adding more countries.”
[I bet you wanna know what he’s talking about. Stay tuned.]
LinkedIn Member 2: “Don't you find it encouraging there are more and more online tools available to us?”
[Who is this sickening optimist?]
LinkedIn Member 1: “I do find it encouraging. What's the best way to keep up with all these new tools? Is there a web site keeping track of them?”
Ok, I’m LinkedIn Member 2, and the short answer to the question about tracking is "no". But I've been maintaining a laundry list of useful regulatory websites and online tools for myself, so thanks to this discussion, I scored my first blog topic for 2015. In no particular order, here are some of the tools/sites on my list. (The first in the list is the subject of the LinkedIn discussion above.)
ClinRegs (NIH’s National Institute of Allergies and Infectious Diseases [NIAID])
 NIAID designed ClinRegs to help researchers plan and execute international clinical trials. The online database consolidates clinical research regulatory requirements for 14 countries, and enables easy comparisons among them. Four mouse clicks was all I needed to compare Brazil’s informed consent requirements with those of Peru. The other 12 countries on the website are China, India, Kenya, Liberia, Malawi, Sierra Leone, South Africa, Tanzania, Thailand, Uganda, the UK, and the US. According to NIAID, future additions may include Mali, Mexico, Haiti, and Vietnam.
NIAID designed ClinRegs to help researchers plan and execute international clinical trials. The online database consolidates clinical research regulatory requirements for 14 countries, and enables easy comparisons among them. Four mouse clicks was all I needed to compare Brazil’s informed consent requirements with those of Peru. The other 12 countries on the website are China, India, Kenya, Liberia, Malawi, Sierra Leone, South Africa, Tanzania, Thailand, Uganda, the UK, and the US. According to NIAID, future additions may include Mali, Mexico, Haiti, and Vietnam. ResearchAE.com (Social Health Insights)
 In June, subscribers to our newsletter read this rather enthusiastic announcement:
In June, subscribers to our newsletter read this rather enthusiastic announcement:”While you're strolling the pharmacy aisles, or talking to your doctor about a prescription, wouldn't it be helpful to know about the adverse events associated with a drug, the demographics of people who experienced them, and what they were taking the drug for? Now you can.”
I’m still enthusiastic. ResearchAE.com makes it easy to search and analyze millions of drug and medical device adverse events, food and medical device recalls, and more. As an example, from the more than 8 million AE records available in openFDA (FDA’s open data API initiative), in a matter of seconds I was able to find all AEs associated with hospital stays for women my age who took cyclobenzaprine last year for muscle spasms. There was one. That’s quite an improvement over the weeks it could take to get the same information via a Freedom of Information Act request.
Data Dashboard (FDA)
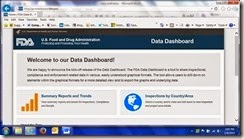 - How many inspections were conducted in Italy in the
- How many inspections were conducted in Italy in thepast 5 years?
- How many warning letters were sent by CDRH in last
year?
- How many Class I food recalls were there in 2010
- What proportion of CDER inspections resulted in
voluntary and official action indications in 2012?
The FDA Data Dashboard can be used to quickly answer any of these questions. High-level data about inspections, warning letters, seizures, injunctions, and recalls are displayed graphically, with options to filter results by year and center. The user can then drill down to view lower levels of detail in tabular form and sort the data by column of interest. Finally, the charts and tables can be exported if further independent analysis is required.
Trends, Charts, and Maps in ClinicalTrials.gov (NIH)
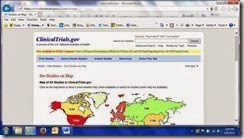 While not nearly as interactive as the previous sites, this page deserves an honorable mention. It provides some interesting data summaries about clinical trials at a glance. Visitors can view charts and diagrams that show the total number of registered studies by type and year, and the number of registered studies with posted results. The map feature that shows global study locations is the sole interactive piece. It’s easy to use and once you’ve drilled down to the country level, a subsequent mouse click will kick off a clinicaltrials.gov query for all studies registered there.
While not nearly as interactive as the previous sites, this page deserves an honorable mention. It provides some interesting data summaries about clinical trials at a glance. Visitors can view charts and diagrams that show the total number of registered studies by type and year, and the number of registered studies with posted results. The map feature that shows global study locations is the sole interactive piece. It’s easy to use and once you’ve drilled down to the country level, a subsequent mouse click will kick off a clinicaltrials.gov query for all studies registered there.It is possible to download raw XML-formatted data directly from ClincalTrials.gov. With that, a developer could build any additional trend analysis required. Since I personally haven’t written a line of code since the Clinton administration, I’ll stick to clicking the interactive map.
Worth Watching
 Just before the new year, FDA announced the availability of a consolidated, searchable database of all guidance documents issued by the agency. Previously, lists of guidance documents were strewn across the FDA website and were inconsistently organized across centers. I’ve always found these lists nearly impossible to navigate (though I admit my fellow Polaris colleagues have fared better). You’d be hearing my celebratory “woot woot” from here if it weren’t for a few tweets I read recently that identified a number of guidance documents missing from the database. There’s speculation that FDA may still be populating it, so I remain hopeful about the database’s usefulness, and will check back periodically to see how it’s progressing.
Just before the new year, FDA announced the availability of a consolidated, searchable database of all guidance documents issued by the agency. Previously, lists of guidance documents were strewn across the FDA website and were inconsistently organized across centers. I’ve always found these lists nearly impossible to navigate (though I admit my fellow Polaris colleagues have fared better). You’d be hearing my celebratory “woot woot” from here if it weren’t for a few tweets I read recently that identified a number of guidance documents missing from the database. There’s speculation that FDA may still be populating it, so I remain hopeful about the database’s usefulness, and will check back periodically to see how it’s progressing.Closing Comments
ClinRegs, ResearchAE, and the FDA Data Dashboard are all in either beta or early release. The developers of these tools are encouraging feedback, so there’s an opportunity to influence these tools to improve their usability and content.
And speaking of encouraging feedback, I’d like to invite you to share any tools you know of that you think others might find helpful. Thank you, readers, and happy new year.
No comments:
Post a Comment